Novel Methods for Inhibiting Amyloidogenesis in the Presence of Peptides to Block Hydrophobic Interactions
Abstract
Amyloid fibrils, which are caused by abnormal conformation and the mis-assembly of proteins, are responsible for several conformational diseases, including prion diseases. To develop methods to prevent amyloid formation, blocking peptides with hydrophilic substitutions covering the stem forming regions of barnase 1-24 were prepared and examined for their ability to block amyloid-forming fragments—prion, Amyloid β, Pmel 17—. When these fragments were mixed with the synthetic blocking peptides, the result was a decline in the intensity of fluorescence, suggesting that amyloid formation was inhibited. Therefore, amyloidogenesis appears to be specifically inhibited by disrupting the hydrophobic interactions between core amyloid regions.
Author Contributions
Copyright © 2024 Masatoshi Saiki, et al
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
The formation of amyloid fibrils is responsible for several disorders including Alzheimer’s disease, prion disease, and dialysis amyloidosis, which are collectively known as “conformational diseases” 1. Amyloid fibrils play a role in many neurodegenerative diseases 2. On the other hand, functional polymers similar to amyloid-like fibrils are formed by the transmembrane protein Pmel17 in melanosomes 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13. Although there is wide variation in the native three-dimensional structures, protein-forming amyloids are typically 10 nm in diameter, long, and unbranched 14, 15, 16, 17, 18, 19, and can be identified by their unique ability to bind to the dyes thioflavin T (ThT) and Congo red. The fibril structure has recently been clarified in detail by cryo-electron microscopy 20.
Insight into mechanisms that inhibit amyloidogenesis is crucial for the therapeutic treatment and clinical analysis of amyloid-related disease 21. Recently, Griner et al. 22 designed peptide-based inhibitors that reduce both amyloid β (Aβ) aggregation and the toxicity of aggregated species. Despite the common morphology of amyloid fibrils, however, there is no general inhibitor of amyloidogenesis.
To develop methods for preventing amyloid formation, it is necessary to inhibit the aggregation of β-structures 23, 24. Recently, it was shown the approach to bind such segments in β-strand and β-hairpin conformations using de novo designed scaffolds 25. Previously, we examined intermolecular interactions among several amino acid residues in barnase1-24 (BM1-24), a protein known to form amyloid-like fibrils 26. Using a series of mutated barnase molecules, we identified interactions between hydrophobic residues on both sides of the β-strand (Figure 1a-c) that are essential in fibril formation. In the present study, to block the hydrophobic interactions between hydrophobic residues on both sides of a β-strand, two peptides (SS-1, SS-2) were prepared in which residues on only one side of the β-strand, located at even numbers, were substituted by hydrophilic residues (Figure 1d-f). A negative control (NC) peptide, possessing hydrophobic residues on both sides of the β-strand, was also prepared.
Figure 1.Model summarizing the strategy for preventing amyloid formation by blocking interactions in amyloid core regions. Dark gray balls represent hydrophobic residues. (a) Oblique projection of part of an amyloid fibril. (b) Antiparallel β-sheet with an even-length of β-strands for amyloid formation. (c) Cross-sectional view of an amyloid fibril. Dotted lines represent hydrophobic interactions between neighboring protofibrils. (d) Designed β-strand for blocking amyloid formation. (e) Cross-sectional view showing the blocking amyloid formation. (f) Amino acid sequences of the synthetic peptides. The sequence of BM1-24 is given in the first row. The special sequence, DA* , where A* denotes D-Ala, is designed to induce a turn. Hydrophobic residues are highlighted in gray.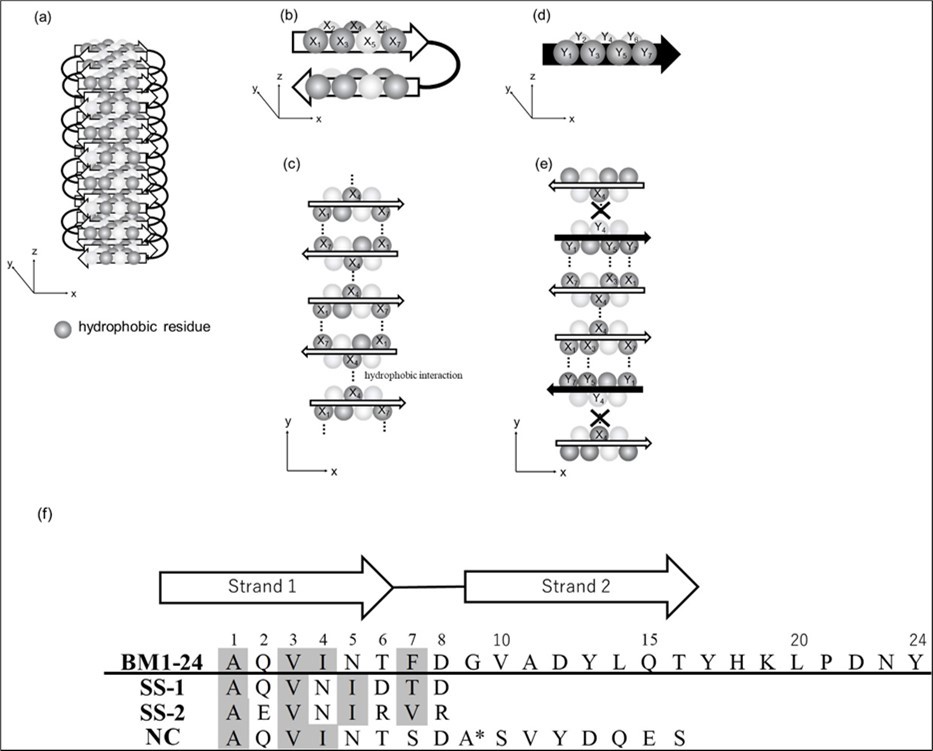
Materials and methods
Peptide synthesis
Peptides were prepared by solid-phase peptide synthesis using Fmoc strategy as previously described 27. In brief, peptides with a C-terminal amide group were assembled on Fmoc-NHCH2 Ph(OCH3)2-O -resin obtained from Rapp Polymere. The subsequent coupling of Fmoc-protected amino acids was carried out using 2-(1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium tetrafluoroborate (TBTU) and N-hydroxybenzotriazole (HOBt). In each synthetic cycle, the terminal Fmoc group was removed by DMF solution containing 1.1% 1,8-diazabicyclo (5.4.0)-7-undecene, 7.7% piperidine, and 2.3% 1-hydroxybenzotriazole. The protecting group and resin were removed by shaking the peptidyl resin in trifluoroacetic acid containing 5% triisopropylsilane and 3% water for 1.5 h at room temperature. The reaction mixture was poured into cold diethyl ether, and the precipitated peptide was collected. The product was taken up by dissolving the mixture in 20% aqueous solution of acetic acid and filtering the mixture to remove the resin beads. RP-HPLC was used to purify the peptides.
Fibril formation
Each lyophilized synthetic peptide (SS-1, SS-2, and NC) was solubilized at 0.2 mM in 50 mM Tris chloride buffer (pH 7.5) in a microtube. Lyophilized peptides of BM1-24, prion180-193, Amyloid β, and serum amyloid A protein 1-27 (SAA1-27) were prepared in the same solution, whereas Pmel17 405-420 was solubilized in sodium acetate buffer (pH 6.0). These solutions were admixed with SS-1, SS-2, or NC, incubated for 7 d under static conditions at 4°C, and then analyzed by ThT assay and CD spectroscopy.
Thioflavin T assay
The thioflavin T (ThT) assay was used for the detection of fibril formation by measuring ThT fluorescence enhancement that occurs in the presence of fibrils. Synthetic peptides were prepared by adding 20 mL of incubated peptide solution to 2 mL of aqueous ThT. The final concentrations of the peptide and ThT were 2 mM and 5 mM, respectively. The formation of amyloid fibrils was monitored by fluorescence enhancement of fibril-bound ThT in 50 mM Tris buffer (pH 7.5). Fluorescence emission spectra were collected in the range 460–600 nm with an excitation wavelength of 450 nm as previously described 13. Fluorescence enhancement of ThT in the amyloid-bound state, ΔF, was defined as ΔF = (FS − F0) / F0, where FS and F0 denote the fluorescence intensity of the sample and that of the control solution without peptides, respectively.
Circular dichroism spectroscopy
A circular dichroism (CD) spectrum was recorded in the far-UV region (200–260 nm) at 20°C with a JASCO J-725 spectropolarimeter, a quartz cuvette, and a 1.0 mm path length. The spectral data were recorded in terms of mean residue ellipticity, (θ), in degrees square centimeter per decimole.
Results and Discussion
Peptide Inhibition of BM1-24 Amyloidogenesis
The ability of BM1-24 to form fibrils in the presence or absence of the synthetic blocking peptides (SS-1 and SS-2) and the negative control peptide (NC) was evaluated by ThT assay, as shown in Figure 2. Based on a previous report 26, 27, 28, a change in fluorescence intensity (ΔF) of >1 was considered to indicate a significant amount of amyloid formation. The solution of BM1-24 alone underwent considerable amyloid formation, as shown by the ΔF value of >3. In contrast, adding SS-1 pr SS-2 to the BM1-24 solution resulted in much a lower fluorescence intensity (ΔF < 0.5), suggesting that amyloid formation was inhibited. Furthermore, the NC peptide comprising hydrophobic residues on both sides of the β-strand was unable to inhibit amyloid formation, suggesting that one side of the BM1-24 b-strand is formed by hydrophilic residues.
Figure 2.Thioflavin-T assays showing amyloid formation of BM1-24 and the mixture of BM1-24 with synthetic blocking peptides.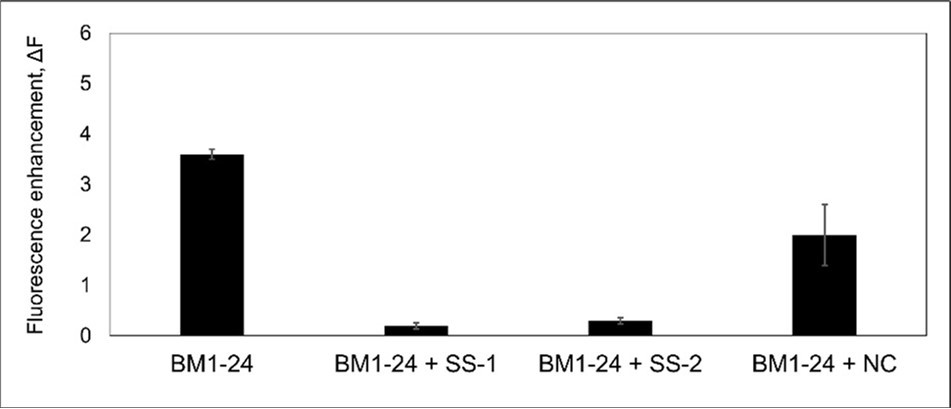
To obtain structural information related to fibril formation of BM1-24 in the presence and absence of SS-1, we carried out CD measurements under the same biochemical conditions as the ThT assay (Figure 3). No significant secondary structure was observed in the SS-1 peptide. By contrast, BM1-24 exhibited a characteristic CD pattern with a negative peak at 207 nm, as reported previously 26, 29. In addition, BM1-24 in the presence of SS-1 produced a CD spectrum with a negative peak at 218 nm, which is consistent with a β-sheet-like structure. It is possible, therefore, that addition of the SS-1 peptide to BM1-24 may have stabilized the β-structure of BM1-24 with hydrophobic residues of SS-1 being specifically recognized by hydrophobic residues of BM1-24.
Figure 3.CD spectra of SS-1, BM1-24, and BM1-24 in the presence of SS-1. Dashed line, SS-1; dotted line, BM1-24; solid line, BM1-24 plus SS-1.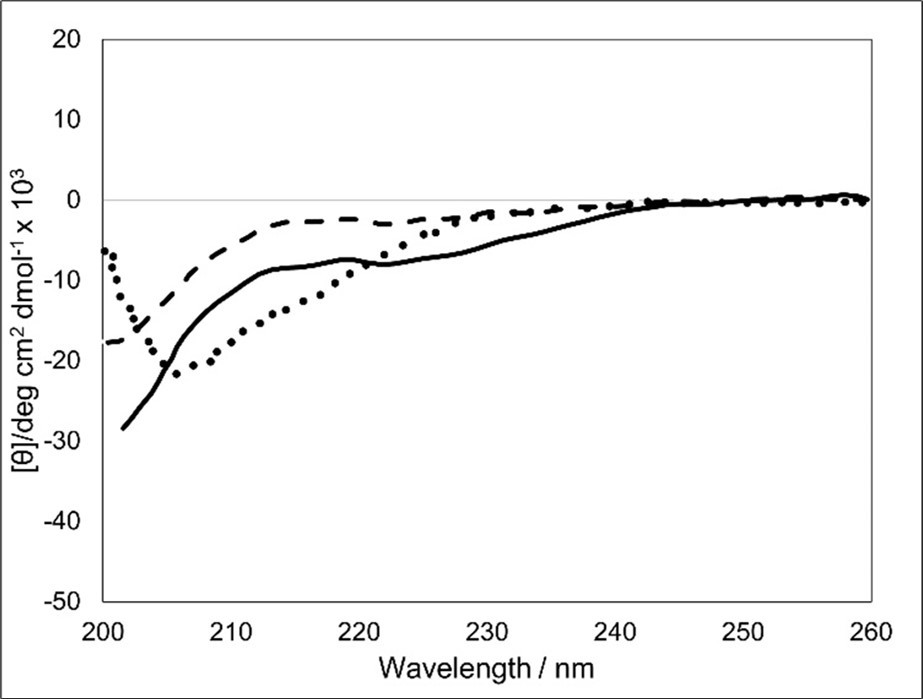
Peptide Inhibition of General Amyloid genesis
Next, we investigated whether the SS-1 peptide might be applicable to the inhibition of other proteins that are known to form amyloid fibrils. We prepared peptides of the following amyloid-forming proteins, prion180-193 27, Amyloid β, serum amyloid A (SAA) protein 1-27, and Pmel17 405-420 30 by chemical synthesis and assessed amyloidogenesis in the presence of SS-1 by ThT fluorescence assay. As shown in Figure 4, addition of SS-1 to each of the amyloid-forming fragments led to a decline in fluorescence intensity, suggesting that amyloid formation had been inhibited. Therefore, our results indicate that the SS-1 peptide is effective at inhibiting amyloidogenesis in various types of protein through the formation of hydrophobic interactions.
Figure 4.Thioflavin-T assays of amyloid fragments in the absence (open bars) and presence (shaded bars) of SS-1.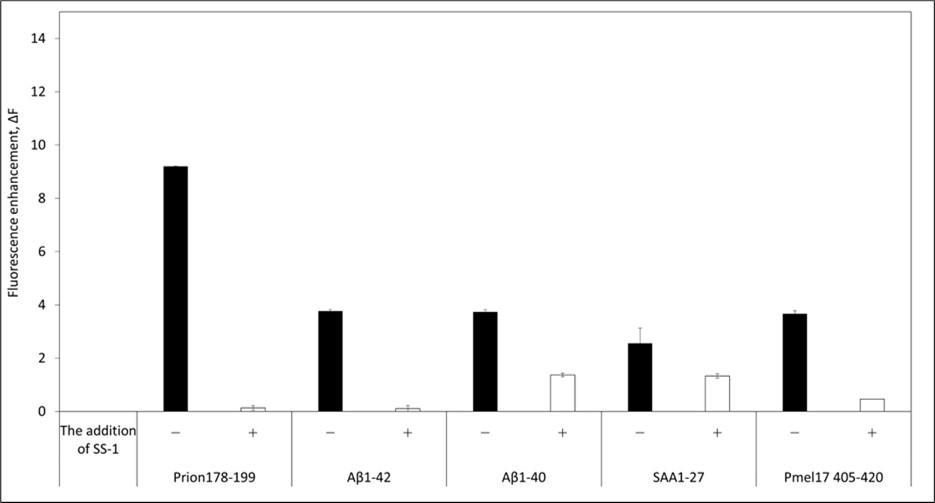
Conclusions
Herein, we have presented a structural model to design peptides for blocking amyloi-dogenesis via the formation of hydrophobic interactions. The SS-1 peptide, comprising eight amino acids, was designed to have hydrophilic residues (Gln, Glu, Asn, and Asp) on only one side of the β-sheet. In addition to inhibiting amyloid formation by BM1-24, SS-1 blocked amyloidogenesis by peptides of prion protein, amyloid-β, SSA, and Pmel17. In summary, amyloidogenesis seems to have been specifically inhibited by the disruption of hydrophobic interactions between core amyloid regions. Our newly designed peptide may be useful for analyzing amyloid β aggregate formation and for studying diseases associated with the formation of amyloid fibrils (amyloidosis). Further optimizing the design of inhibitory peptides and verifying their effectiveness in in vivo experiments will likely lead to the development of more effective amyloid fibril inhibitors.
Abbreviations
References
- 2.Wojciech P, Vincenza A Umesh, Slawomir F. (2012) . Ubiquitous Amyloids.Applied Biochemistry and Biotechnology166 1626-1643.
- 3.Berson J F, Harper D C, Tenza D, Raposo G, Marks M S. (2001) Pmel17 initiates premelanosome morphogenesis within multivesicular bodies.Mol. , Biol. Cell.123451–3464
- 4.Fowler D M, Koulov A V, Alory-Jost C, Marks M S, Balch W E et al. (2006) Functional amyloid formation within mammalian tissue.PLOS Biol.4e6.
- 5.McGlinchey R P, Shewmaker F, McPhie P, Monterroso B, Thurber K et al. (2009) The repeat domain of the melanosome fibril protein Pmel17 forms the amyloid core promoting melanin synthesis.Proc. , Natl Acad. Sci. U. S. A.10613731–13736
- 6.McGlinchey R P, Shewmaker F, Hu K N, McPhie P, Tycko R et al. (2011) Repeat domains of melanosome matrix protein Pmel17 orthologs form amyloid fibrils at the acidic melanosomal pH.Journal of Biological Chemistry286. 8385-8393.
- 7.Dean D N, Lee J C. (2019) pH-Dependent fibril maturation of a Pmel17 repeat domain isoform revealed by tryptophan fluorescence.BiochimicaetBiophysicaActa (BBA) - Proteins and. Proteomics1867 961-969.
- 8.Dean D N, Lee J C. (2020) Modulating functional amyloid formation via alternative splicing of the premelanosomal protein PMEL17.Journal of Biological Chemistry22. 7544-7553.
- 9.Dean D N, Lee J C. (2021) Purification and characterization of an amyloidogenic repeat domain from the functional amyloid Pmel17.Protein Expression and. Purification187 105944.
- 10.Watt B, Niel G, Fowler D M, Hurbain I, Luk K C et al. (2009) N-terminal domains elicit formation of functional. Pmel17 amyloid fibrils.Journal of Biological Chemistry284 35543-35555.
- 11.Louros N N, Iconomidou V A. (2016) Identification of an amyloid fibril forming segment of human. Pmel17 repeat domain (RPT domain).Biopolymers106 133-139.
- 12.Harper D C, Theos A C, Herman K E, Tenza D, Raposo G et al. (2008) Premelanosome amyloid-like fibrils are composed of only golgi-processed forms of Pmel17 that have been proteolytically processed in endosomes.Journal of Biological Chemistry283. 2307-2322.
- 13.Pfefferkorn C M, McGlinchey R P, Lee J C. (2010) Effects of pH on aggregation kinetics of the repeat domain of a functional amyloid. Pmel17.Proceedings of the National Academy of Sciences107 21447-21452.
- 14.Sunde M, Serpell L C, Bartlam M, Frasera P E, Pepysa M B et al. (1997) Common core structure of amyloid fibrils by synchrotron X-ray diffraction.J. , Mol. Biol.273729–739
- 15.Kelly J W. (1996) Alternative conformations of amyloidogenic proteins govern their behavior.Curr.Opin. , Struct. Biol.611–17
- 17.Chiti F, Dobson C M. (2009) Amyloid formation by globular proteins under native conditions.Nat. , Chem. Biol.515–22
- 18.Prusiner S B. (1996) Human prion diseases and neurodegeneration.Curr. Top.Microbiol. Immunol.2071–17
- 19.Kent R T, Wai-Ming Y, Robert T. (2024) Structure of Amyloid Peptide Ribbons Characterized by Electron Microscopy, Atomic Force Microscopy, and Solid-State Nuclear Magnetic Resonance.J. , Phys. Chem 128, 1711-1723.
- 20.Gremer L, Schölzel D, Schenk C, Reinartz E, Labahn J et al. (2017) Fibril Structure of amyloid-β(1-42) by Cryo-Electron Microscopy.Science358116–119.
- 21.Ly L, Parsons R, Austen B. (2020) . Purification of Full-Length β-Secretase Involved in Alzheimer’s Disease, and Proteomic Identification of Binding Partners.Journal of Biomedical Science and Engineering13 1-12.
- 22.Griner S L, Seidler P, Bowler J, Murray K A, Yang T P et al. (2019) Structure-based inhibitors of amyloid beta core suggest a common interface with tau.eLife.8e46924–e46952.
- 23.S V Armiento. (2020) Spanopoulou A and Kapurniotu A. Peptide-Based Molecular Strategies To Interfere with Protein Misfolding, Aggregation, and Cell Degeneration.AngewandteChemie59, 3372 – 3384.
- 24.Pellegrino S, Tonali N, Erba E, Kaffy J, Taverna M et al. (2017) β-Hairpin mimics containing a piperidine–pyrrolidine scaffold modulate the β-amyloid aggregation process preserving the monomer species.Chemical. Gelmia ML and Ongeri S 1295-1302.
- 25.Danny D S, Ewa A A, Hannah L H, Enrico R, Matthias M S et al. (2024) Design of amyloidogenic peptide traps. Nat Chem Biol. Online ahead of print.
- 26.Saiki M, Honda S, Kawasaki K, Zhou D, Kaito K et al. (2005) Higher-order molecular packing in amyloid-like fibrils constructed with linear arrangements of hydrophobic and hydrogen-bonding side-chains.J. , Mol. Biol.348983–998
- 27.Saiki M, Shiba K, Okumura M. (2015) Structural stability of amyloid fibrils depends on the existence of the peripheral sequence near the core cross-b region.FEBS Lett.5893541–3547.
- 28.Morii H, Saiki M, Konakahara T, Ishimura M. (2006) Peripheral region for core cross-beta plays important role in amyloidogenicity.Biochemical and Biophysical Research. Communications342 808-816.
