Low Seroprevalence of Brucella Spp. among Remote Colombian Communities from the Sierra Nevada De Santa Marta
Abstract
Brucella species cause a high burden of disease globally, infecting both humans and animals; however, One Health has been under-appreciated in Colombia. This study aimed to determine the seroprevalence of Brucella spp. in two remote indigenous communities from the Sierra Nevada de Santa Marta, Colombia. These communities live in close contact with their livestock, indicating a potential susceptibility to zoonotic pathogens. The livestock routinely kept by these communities include cattle, small ruminants and pigs, the known hosts of human pathogenic Brucella spp.. A low level of exposure to Brucella spp. was documented, with only one positive participant among 539 participants (0.2%; 95% CI 0.0 – 1.0). Nevertheless, due to the high risk that zoonoses pose, we recommend discussions with the community for the potential establishment of One Health surveillance studies for the early detection and prevention of future zoonotic disease threats.
Author Contributions
Academic Editor: Anoja Priyadarshani, Senior Lecturer in Biochemistry, Department of Biochemistry, Faculty of Medicine, University of Ruhuna, Sri Lanka
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2023 Regina oakley, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Brucella species are gram negative bacteria causing brucellosis, a zoonotic disease of global significance1. The species most often involved in human infection are B. melitensis, B. abortus, B. suis, and B. canis2. The most common clinical findings in human brucellosis are fever and a flu-like illness, followed by osteoarticular manifestations3. Lymphadenopathy and a broad palette of genitourinary, neurological, pulmonary and gastrointestinal complications are also possible3,4. Human brucellosis has an overall mortality rate of 5%, most commonly linked to endocarditis3. In livestock, brucellosis causes abortion, infertility and subsequent loss of milk production5. In addition to the high disease burden in animals and humans, brucellosis can cause substantial economic impact due to reduced animal production and patient time lost from work and routine activities6,7. B. abortus and B. melitensishave the greatest economic impact5. Animal to human transmission occurs through direct contact with placenta, amniotic fluid or aborted foetuses or during slaughtering of infected animals, as well as through consumption of unpasteurized dairy products from infected animals4. In Latin America, Brucella spp. have been isolated from humans, domestic animals (cattle, goats, pigs, sheep, dogs and horses) as well as wildlife (buffalos, foxes, grey weasels, capybaras and ferrets)8. Most human isolates have come from Argentina, Peru and Mexico; whereas in Colombia, Brazil, Chile and Cuba, Brucella spp. was predominantly isolated from cattle8.
There are limited reports on the seroprevalence of Brucella spp. in humans from South America. One study on the population of an urban slum in Brazil found sero-positivity of 13% for B. abortus and 4.6% for B. canis9. A study from Northwest Ecuador found an overall seroprevalence of only 1.9%10. Although, the seroprevalence for participants in the high-risk group (occupational exposure to animals) was 4.8% compared to 1.4% in the low-risk group (no occupational exposure to animals)10.
Serological studies in animals from South America have primarily been conducted in Argentina and Brazil. In Argentina, Brucella spp. exposure has been reported in dogs (14.7%)11, cattle (3.7%)12, water buffalo (6.4%)13, foxes (17.8%)14, minks (9.2%)15 and armadillos (16.0%)16. In Brazil, Brucella spp. exposure has been reported in cattle (2.2% - 3.7%), buffaloes (4.8% - 6.8%)17,18, equines (1.3%)19 and rams (2.9%)20,21. One additional study from Ecuador also reported exposure in cattle (16.7%)22,23.
In this study we hypothesized to find a high seroprevalence of Brucella spp. in the two indigenous communities, the Wiwa and Koguis, residing in the Sierra Nevada de Santa Marta, Colombia. Due to the limited contact these communities have with the outside world, little is known about their healthcare needs and disease burden. It was expected that these communities would have a high burden of infectious disease due to their limited access to medical services, poor socioeconomic status, inadequate access to sanitation and clean drinking water and simple housing (clay huts with unsealed floors)24,25. These communities live in close contact with their livestock (cattle, small ruminants, pigs and poultry), indicating a potential susceptibility to zoonotic pathogens including Brucella spp.
Materials and Methods
Study population and data collection
This cross-sectional study used a subset of 539 serum samples and demographic data collected within a larger research study called “Colombia-Germany research program on diagnostics, research, treatment and prevention of Chagas Disease (CD) and emerging infectious diseases in vulnerable groups” between 2021 and 2022. Samples were collected from two indigenous communities, the Wiwa and Koguis whose population was reported as 18,202 and 15,820, respectively, in the 2018 census26. The samples were randomly selected from the larger study and stratified by village. The Wiwa participants (n = 264) came from five villages: Surimena (n = 52), Ahuyamal (n = 64), Sabannah de Higueron (n = 68), Dungakare (n = 36) and Potrerito (n = 43). The Koguis participants (n = 275) came from four villages: Zarachui (n = 94), Mamangueka (n = 63), San Jose (n = 114), Avingue (n = 4) (Figure 1). Exposure data for common zoonotic disease risks was also collected for 147 of the included Wiwa participants.
Figure 1.Map of the Sierra Nevada de Santa Marta, Colombia. Included Wiwa and Koguis villages are located within the area marked by the black oval. Insert shows a map of Colombia with the Sierra Nevada de Santa Marta highlighted in red. Adapted from Google Maps and Vemaps28,29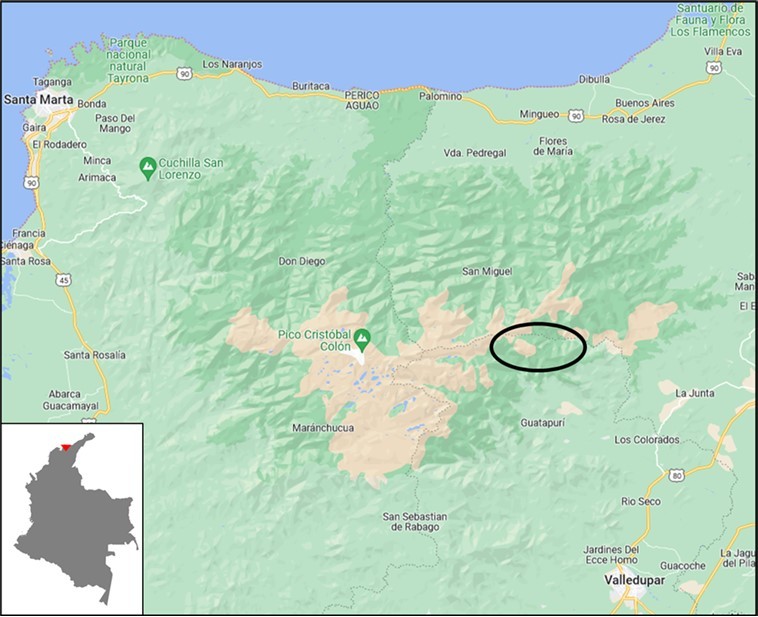
Community members were invited to participate in the study after being informed about the project. Volunteers aged ≥ 12 years were recruited into the study if they had a positive Chagas rapid diagnostic test (RDT) result, while all volunteers < 12 years old were recruited to avoid an additional blood draw required for the Chagas RDT. Blood samples were collected with a vacutainer (8ml) and kept in a cooler at 4°C (provided by World Courier Frankfurt, Germany) in the field before being transferred daily to the laboratory where they were centrifuged and stored at -20°C27. Samples were shipped on dry ice to Medmissio, Würzburg, Germany (World Courier Frankfurt, Germany) where they were stored at −80 °C27
Sample size calculation
To determine the seroprevalence of Brucella spp. in the Wiwa and Koguis people from Colombia, a required sample size of 196 participants was calculated using epitools from https://epitools.ausvet.com.au30. The sample size was calculated for a precision of 0.05, a confidence of 0.95 and an estimated Brucella spp. seroprevalence of 15%. Since no information regarding the seroprevalence of Brucella spp. in humans in Colombia was found, this conservative estimate was based on the higher seroprevalence found in the Brazilian study9.
Ethical considerations
Written informed consent (or witnessed thumb print) was obtained from all participants - or legal guardians of a child where appropriate - prior to sample collection. The studies were performed in accordance with the principles of the Declaration of Helsinki and were approved by the Institutional Ethic Committee for Investigation of Bogota, Colombia (Acta No. 2019-4). Ethical approval and authorization to perform the study was also granted by the Governors of the Wiwa and Kogius communities with permission to enter their territory.
Serological testing
Serological testing was performed at the Swiss Tropical and Public Health Institute, Basel Switzerland. The serum samples (n=539) were screened by the Serion enzyme-linked immunosorbent assay (ELISA) Brucella IgG classic (Serion/Verion Immundiagnostica GmbH, Würzburg Germany) according to the manufactures instructions with the positivity cut-off determined using the manufacturers automated software31. The software uses a 4-parameter logistic function to establish a lot-specific standard curve to determine the test result using standard serum included in each run31. An additional cut off was set by calculating the mean of the optical density (OD) plus three standard deviations. Serum samples with an OD over this additional cut off were sent to the Institute for Infectious Diseases (IFIK), University of Bern (Bern, Switzerland) for confirmation by the BrucellaCapt test (Vircell, Granada, Spain).
Results
The samples included were from participants aged from 1-90 years, with a median age of 22 years (IQR 11 - 37), with 57% being female. Of the 539 serum samples screened by the Serion ELISA, one sample was ELISA positive and one was equivocal. In the screening phase, thirteen samples (including the positive and equivocal sample from the Serion ELISA) demonstrated an OD greater than the additional cut-off calculated based on the negative results. These 13 samples underwent confirmatory testing by the BrucellaCapt test (performed at the certified laboratory IFIK, Bern, Switzerland). One sample, from a Koguis participant was found positive by the BrucellaCapt test – leading to an overall seroprevalence of 0.2% (95% CI 0.0 – 1.0).
Exposure data in the Wiwa people (n = 147) identified specific practices associated with zoonotic disease transmission: animal slaughtering (84.4%), assisting with birth (23.8%) and milking (18.4%) including cattle, goats and sheep (Table 1). One investigator reported similar practices among the Koguis people. All Wiwas and Koguis questioned reported regular exposure to and handling of livestock and animals (cattle, goats and sheep), thereby limiting the unequivocal identification of risk factors.
Table 1. Exposure among the Wiwa people (n = 147) living in the Sierra Nevada de Santa Marta, Colombia, to potential risk factors for the transmission of zoonotic pathogens.| Exposure | Positive, n (%) |
| Participate in animal slaughter: | |
| Chickens | 108 (73.5) |
| Turkeys | 5 (3.4) |
| Pigs | 16 (10.9) |
| Cattle | 7 (4.8) |
| Goats | 72 (49.0) |
| Sheep | 4 (2.7) |
| None | 23 (15.6) |
| Assist in animal birth: | |
| Pigs | 2 (1.4) |
| Cattle | 5 (3.4) |
| Goats | 8 (5.4) |
| Sheep | 2 (1.4) |
| Other | 5 (3.4) |
| None | 112 (76.2) |
| Milking of animals: | |
| Cattle | 12 (8.2) |
| Goats | 8 (5.4) |
| Sheep | 6 (4.1) |
| Other | 3 (2.0) |
| None | 120 (81.6) |
Discussion
A very low seroprevalence of <1% was identified for Brucella spp. in over 539 sera from two indigenous communities residing in the Sierra Nevada de Santa Marta, Colombia. While studies are limited on brucellosis in Colombia, two case reports on Brucella spp. infections in humans describe; a farmer with antibodies to Brucella spp. (not speciated) believed to have been infected through contact with cattle32 and a merchant diagnosed with B. melitensis by blood culture, whose infection was suspected to be linked to consumption of unpasteurized milk33. A molecular study in the Colombian Caribbean region found 22.2% (6/27) of cheese samples made from unpasteurized cow milk to be positive for Brucella spp. DNA34. B. abortus is the only species previously identified in livestock from Colombia, with herd-level seroprevalence reported between 22.0% – 27.5%35, 36, 37, 38, 39. The only other species identified in Colombia is B. canis, found in humans (9.0%) and dogs (2.0% - 15.0%) in Bogotá and the Antioquia region40, 41, 42. There is evidence for the absence of Brucella spp. exposure in regions of Colombia in two serological studies; one in domestic pigs (n = 350, in 23 farms), and another in collared peccaries (Pecari tajacu, n = 58) and feral pigs (n = 15) finding no seropositivity for Brucella spp.43,44.
The serion ELISA used in our study is coated with native extract from B. abortus, containing genus-specific antigens for the detection of antibodies against human pathogenic Brucella spp31. The manufacturer reported a high sensitivity (>99%) and specificity (99.3%)31. An independent evaluation, however, found a lower sensitivity of 84%, subsequently there is a possibility of false negatives with the ELISA45. Although, we do not believe this to affect the findings of this study as the samples with the highest ELISA OD readings were confirmed negative by the BrucellaCapt test performed at the certified IFIK, Bern, Switzerland. The BrucellaCapt test covers the three smooth lipopolysaccharides (LPS) containing human pathogenic Brucella spp. (B. melitensis, B. abortus and B. suis) with a reported 100% sensitivity and specificity45,46. B. canis, however, has a rough LPS and is often not adequately diagnosed by routine serological tests47. The reports of B. canis in Colombia may warrant further epidemiological investigations to determine the exposure to dogs and B. canis among the indigenous tribes of the Sierra Nevada de Santa Marta.
While we found limited evidence of Brucella spp. amongst the Wiwa and Koguis tribes, this should not discount the potential risk of zoonotic diseases of these remote, vulnerable communities, with notably highly limited access to health care facilities.
Zoonotic diseases are responsible for substantial morbidity and mortality, particularly in low resource communities with high dependence on livestock48. Globally, zoonoses account for 60.3% of emerging infectious diseases48. The major drivers of their emergence or re-emergence include expansion of cities or farmland with deforestation, increasing human population and urbanization, globalization of food systems, climate change and environmental contamination48, 49, 50. Remote tribes having been protected through their isolation and with high proportions of immunologically naïve inhabitants may be particularly vulnerable to these drivers and exposure to new pathogens. Given the high level of exposure to animals in the populations of this study, it was unexpected to find such a low seroprevalence for this normally common zoonotic disease. The low rates could be attributed to the isolated raising and maintenance of livestock with small herd numbers, reducing the contact rate and transmission risk51. Introduction of infected animals poses a risk to these vulnerable populations and discussions with the community on potential surveillance programs are warranted. One Health surveillance systems provide a means of early detection to prevent outbreaks in both animal and human hosts52. These systems are particularly effective for zoonoses that have limited human-to-human transmission risk, as is the case with brucellosis, which can be detected and controlled in the animal host preventing transmission to humans53,54.
A limitation of this study is that despite testing more than double the calculated sample size, the sample may not reflect adequately the geographical distribution in the Sierra Nevada de Santa Marta, Colombia. The Wiwa and Koguis participants accounted for 1.5% and 1.7% of their entire populations, respectively – and the sampled villages were located to the East of the Sierra Nevada de Santa Marta. We cannot discount the possibility of differential exposure in other Wiwa and Koguis villages. Further, is that we do not have the risk factor data for the Koguis participants, including the Brucella spp. positive individual. Consequently, we cannot comment on the possible route by which this individual became exposed to the bacteria.
Conclusion
The evidence of Brucella spp. exposure in the Wiwa and Koguis tribes was found to be very small with a seroprevalence of <1% by screening/confirmatory testing. Consultation with these communities for the implementation of systematic One Health investigations for the detection and prevention of potential emerging zoonotic threats are recommended due to their isolation and limited access to healthcare.
Acknowledgements
This project was funded by Else Kröner-Fresenius-Stiftung (2019_HA163), and the Stanley Thomas Johnson Foundation (1053-KF).
References
- 1.McDermott J, Grace D, Zinsstag J. (2013) Economics of brucellosis impact and control in low-income countries. doi: 10.20506/rst.32.1.2197. , Rev Sci Tech 32, 249-261.
- 2.A, Marzouk M, Abalkhail E, Almuzaini A, M A. (2023) . The Development of Diagnostic and Vaccine Strategies for Early Detection and Control of Human Brucellosis, Particularly in Endemic Areas. doi: 10.3390/vaccines11030654. Vaccines , (Basel) 11.
- 3.M P Franco, Mulder M, R H Gilman, H L Smits. (2007) Human brucellosis. , The Lancet Infectious Diseases 7, 10-1016.
- 4.E M Galińska, Zagórski J. (2013) Brucellosis in humans--etiology, diagnostics, clinical forms. , Ann Agric Environ Med 20, 233-238.
- 5.Ducrotoy M J Ammary, K Ait Lbacha, Zouagui H, Mick Z, V. (2015) Narrative overview of animal and human brucellosis in Morocco: intensification of livestock production as a driver for emergence? doi: 10.1186/s40249-015-0086-5. Infect Dis Poverty 4(57).
- 6.F Zinsstag Roth, Orkhon J, Chimed-Ochir D, Hutton G, G. (2003) Human health benefits from livestock vaccination for brucellosis: case study. , Bull World Health Organ 81, 867-876.
- 7.Dean A S Crump, Greter L, Hattendorf H, Schelling J, E. (2012) Clinical manifestations of human brucellosis: a systematic review and meta-analysis. doi: 10.1371/journal.pntd.000192. PLoS. , Negl Trop Dis 6, 1929.
- 8.N E Lucero, S M Ayala, G I Escobar, N R Jacob. (2008) Brucella isolated in humans and animals. in Latin America from 1968 to 2006. doi: 10.1017/s0950268807008795. Epidemiol Infect 136 496-503.
- 9.M O Angel, Ristow P, A I Ko, Di-Lorenzo C. (2012) Serological trail of Brucella infection in an urban slum population in Brazil. doi: 10.3855/jidc.2347. J Infect Dev Ctries.
- 10.J Ron-Garrido Ron-Román, Abatih L, Celi-Erazo E, Vizcaíno-Ordóñez M, L. (2014) Human brucellosis in northwest Ecuador: typifying Brucella spp., seroprevalence, and associated risk factors. doi: 10.1089/vbz.2012.1191. Vector Borne Zoonotic Dis. 14, 124-133.
- 11.López G, S M Ayala, A M Efron, C F Gómez, N E Lucero. (2009) A serological and bacteriological survey of dogs to detect Brucella infection in Lomas de Zamora, Buenos Aires province. , Rev Argent Microbiol 41, 97-101.
- 12.M, A R Vila, M S Beade, Balcarce A, W B Karesh. (2003) Health evaluation of pampas deer (Ozotoceros bezoarticus celer) at Campos del Tuyú Wildlife Reserve. , Argentina. doi:, J Wildl Dis 39, 10-7589.
- 13.Konrad J L Campero, L M Caspe, G S Brihuega, Draghi B, G. (2013) Detection of antibodies against Brucella abortus, Leptospira spp., and Apicomplexa protozoa in water buffaloes in the Northeast of Argentina. , Trop Anim Health Prod doi:, 10-1007.
- 14.Martino P E Montenegro, J L Preziosi, J A Venturini, Bacigalupe C, D. (2004) Serological survey of selected pathogens of free-ranging foxes in southern Argentina, 1998--2001. doi: 10.20506/rst.23.3.1521. , Rev Sci Tech 23, 801-806.
- 15.P E Martino, L E Samartino, N O Stanchi, N E Radman, E J Parrado. (2017) Serology and protein electrophoresis for evidence of exposure to 12 mink pathogens in free-ranging. American mink (Neovison vison) in Argentina. doi: , Vet Q 37, 10-1080.
- 16.M S Kin, Fort M, Echaide S T de, E B Casanave. (2014) . Brucella suis in armadillos (Chaetophractus villosus) from La Pampa, Argentina. doi: 10.1016/j.vetmic.2014.01.039. Vet Microbiol 170 442-445.
- 17.Batista H R Passos, Neto C T S Nunes, O G Sarturi, Coelho C, A P L. (2020) Factors associated with the prevalence of antibodies against Brucella abortus in water buffaloes from Santarém, Lower Amazon region. , Brazil. doi:, Transbound Emerg Dis 67, 44-48.
- 18.Silva da, J B Rangel, Fonseca C P da, Morais A H de, Vinhote E et al. (2014) Serological survey and risk factors for brucellosis in water buffaloes in the state of Pará. , Brazil. doi:, Trop Anim Health Prod 46, 10-1007.
- 19.Dorneles E M Gonçalves, V S Santana, J A Almeida, M V. (2015) Brucellosis in working equines of cattle farms from Minas Gerais State. , Brazil. doi:, Prev Vet Med 121, 380-385.
- 20.G Santos Machado, D V Kohek, Stein I, M C Hein, E H. (2015) Seroprevalence of Brucella ovis in rams and associated flock level risk factors in the state of Rio Grande do Sul. , Brazil. doi:, Prev Vet Med 121, 183-187.
- 21.Minervino A H H Soares, H S Barrêto-Júnior, R A Neves, Morini K A L, C A. (2018) Antibodies against Brucella abortus and leptospira spp. in captive mammels in the states of Pará and Rio do Norte. , Brazil. doi:, J Zoo Wildl Med 49, 10-1638.
- 22.A Guzmán Carbonero, L T García-Bocanegra, Borge I, Adaszek C, L. (2018) Seroprevalence and risk factors associated with Brucella seropositivity in dairy and mixed cattle herds from Ecuador. doi: 10.1007/s11250-017-1421-6. Trop Anim Health Prod. 50, 197-203.
- 23.S Oettinger Moya, Borie S, Flores C, Abalos R, P. (2019) Serologic Survey of Brucella canis and Leptospira spp. in Free-Ranging Wild and Domestic Canids from Tierra del Fuego , Chile. doi:, J Wildl Dis 55, 10-7589.
- 24.A Ruf Dreyfus, M T Goris, Poppert M, Mayer-Scholl S, A. (2022) Comparison of the Serion IgM ELISA and Microscopic Agglutination Test for diagnosis of Leptospira spp. infections in sera from different geographical origins and estimation of Leptospira seroprevalence in the Wiwa indigenous population from Colombia. doi: 10.1371/journal.pntd.0009876. PLoS. , Negl Trop Dis 16, 0009876.
- 25.S Bruennert Kann, Hansen D, Mendoza J, Gonzalez G A C, J. (2020) . High Prevalence of Intestinal Pathogens in Indigenous in Colombia. doi: 10.3390/jcm9092786. J Clin Med 9 2786.
- 26.Departamento El.Administrativo Nacional de Estadística. Resultados del censo national de población y vivienda 2018. (2019) El Departamento Administrativo Nacional de Estadística. , Bogotá, Colombia
- 27.S Dib Kann, J C Aristizabal, Mendoza A, G C Lacouture, H D S. (2022) . Diagnosis and Prevalence of Chagas Disease in an Indigenous Population of Colombia. doi: 10.3390/microorganisms10071427. Microorganisms 10 .
- 30.Sergeant E S G. (2018) Epitools Epidemiological Calculators. Available from http://epitools.ausvet.com.au.
- 31.Institut Virion\Serion GmbH.. SERION ELISA classic Brucella IgA/IgG/IgM, v6 20/11 , Würzburg, Germany .
- 32.Casallas García, C J, Monsalve Villalobos, Arias Villate W, C S et al. (2018) Acute liver failure complication of brucellosis infection: a case report and review of the literature. doi: 10.1186/s13256-018-1576-4 . , J Med Case Rep 12(62).
- 33.Piedrahita D, A J Martinez-Valencia, Rojas Agudelo, L O, Tafur E et al. (2021) Fatal Brucellosis Infection in a Liver Transplant Patient: A Case Report and Review of the Literature. doi: 10.1155/2021/1519288 Case Rep Infect Dis. 1519288.
- 34.Soto-Varela Z E Gutiérrez, Moya C G De, Mattos Y, Bolívar-Anillo R, J H. (2018) Molecular detection of Salmonella spp., Listeria spp. and Brucella spp. in fresh artisanal cheese marketed in the city of Barranquilla: A pilot study. doi: 10.7705/biomedica.v38i3.3677. , Biomedica 38, 30-36.
- 35.Arenas N E Abril, D A Valencia, Khandige P, Soto S, Y C. (2017) Screening food-borne and zoonotic pathogens associated with livestock practices in the Sumapaz region. , Cundinamarca, Colombia. doi:, Trop Anim Health Prod 49, 10-1007.
- 36.L M Avila-Granados, D G Garcia-Gonzalez, J L Zambrano-Varon, A M. (2019) . Brucellosis in Colombia: Current Status and Challenges in the Control of an Endemic Disease. doi: 10.3389/fvets.2019.00321. Front Vet Sci 6 321.
- 37.Cárdenas L, Melo O, Casal J. (2018) Evolution of bovine brucellosis in Colombia over a 7-year period (2006-2012). doi: 10.1007/s11250-017-1395-4. Trop Anim Health Prod 50.
- 38.Cárdenas L, Peña M, Melo O, Casal J. (2019) Risk factors for new bovine brucellosis infections in Colombian herds. , BMC Vet Res 15, 10-1186.
- 39.Ramírez O L H Santos, H A Paulino, Meer P G van der, C S Bautista, J L R. (2022) Cross-sectional study of Brucella spp. using real-time PCR from bovine whole blood in Colombia. , Vet Res Commun doi:, 10-1007.
- 40.Agudelo-Flórez P, Castro B, Rojo-Ospina R, Henao-Villegas S. (2012) Canine brucellosiss: Seroprevalence and risk factors in pets from eleven neighbourhoods in Medellin, Colombia. Rev Salud Publica (Bogota). 14, 644-656.
- 41.Castrillón-Salazar L, C A Giraldo-Echeverri, M, Olivera-Angel M. (2013) Factors associated with Brucella canis seropositivity in kennels of two regions of Antioquia. , Colombia. doi:, Cad Saude Publica 29, 10-1590.
- 42.A J Laverde, Restrepo-Botero D, Hernández-Pulido D, J L Rodríguez-Bautista, I S Sandoval. (2021) Seroprevalence of Brucella canis in canines from a dog shelter in. , Bogotá, Colombia. doi:, Biomedica 41, 260-270.
- 43.Montenegro O L Roncancio, Soler-Tovar N, Cortés-Duque D, Contreras-Herrera J, J. (2018) Serologic survey for selected viral and bacterial swine pathogens in Colombian collared peccaries (Pecari tajacu) and feral pigs (Sus scrofa). doi: 10.7589/2017-09-236. , J Wildl Dis 54, 700-707.
- 44.Pulido-Villamarín A P Santamaría-Durán, A N Castañeda-Salazar, Chamorro-Tobar R, Carrascal-Camacho I, K A. (2020) Assessment of antibodies against three zoonotic bacteria and associated risk factors in pig farms in Colombia. doi: 10.20506/rst.39.3.3188. , Rev Sci Tech 39, 923-945.
- 45.Gómez M C Nieto, J A Rosa, Geijo C, Escribano P, A M. (2008) Evaluation of seven tests for diagnosis of human brucellosis in an area where the disease is endemic. , Clin Vaccine Immunol 15, 10-1128.
- 46.G Di Bonaventura, Angeletti S, Ianni A, Petitti T, Gherardi G. Microbiological Laboratory Diagnosis of Human (2021) Brucellosis: An Overview. doi: 10.3390/pathogens10121623. , Pathogens 10.
- 47.Dahouk Al, S, Nöckler K. (2011) Implications of laboratory diagnosis on brucellosis therapy. doi: 10.1586/eri.11.55. Expert Rev Anti Infect Ther 9. 833-845.
- 48.Jones K E Patel, N G Levy, M A Storeygard, Balk A, D. (2008) Global trends in emerging infectious diseases. doi: 10.1038/nature06536. , Nature 451, 990-993.
- 49.N D Wolfe, C P Dunavan, Diamond J. (2007) Origins of major human infectious diseases. doi: 10.1038/nature05775. , Nature 447, 279-283.
- 50.Petersen E, Petrosillo N, Koopmans M. (2018) Emerging infections-an increasingly important topic: review by the Emerging Infections Task Force. doi: 10.1016/j.cmi.2017.10.035. , Clin Microbiol Infect 24, 369-375.
- 51.A J Meadows, C, M J Keeling, M J Tildesley. (2018) Disentangling the influence of livestock vs. farm density on livestock disease epidemics. , Ecosphere 9, 02294.
- 52.J Kaiser-Grolimund Zinsstag, Heitz-Tokpa A, Sreedharan K, Lubroth R, J. (2023) Advancing One human-animal-environment Health for global health security: what does the evidence say? doi: 10.1016/s0140-6736(22)01595-1. , Lancet 401, 591-604.
